The Centers for Medicare & Medicaid Services (CMS) recently released the CY 2026 Medicare Physician Fee Schedule (MPFS) proposed rule, which included significant proposals for remote therapeutic monitoring (RTM). These proposals reflect the expanding recognition of RTM as an important tool to incentivize increased visibility into patient recovery outside clinical settings as well as CMS attempts to align reimbursement policy with how digital care is being delivered in practice.
Below is a summary of the proposals relating specifically to musculoskeletal (MSK) RTM.
Aligning RTM Codes with Real-World Practice
Introduced by CMS in 2022, RTM codes were intended to enable reimbursement for monitoring of patient-reported non-physiological data. However, many providers found some of the requirements to qualify for RTM onerous and unsuitable for a wide range of conditions, contending that many patients may engage with digital recovery and adherence in segmented bursts rather than continuous unbroken streams.
In particular, existing RTM codes (namely 98972, 98977, 98980, and 98981) required at least 16 days of data transmission in each 30-day period and necessitated at least 20 minutes of provider monitoring time per month; for many providers, these thresholds were viewed as arbitrary and excessive for different conditions, and many believed that lower thresholds for data transmission and shorter periods of monitoring would still be clinically valid for various conditions and would avoid risking a lack of coverage for provider time spent supporting patients remotely.
As a result of this feedback, in September 2024, the Current Procedural Terminology (CPT) Editorial Panel added new RTM codes for 2 to 15 days of data transmission within a 30-day period as well as for the first 10 minutes of provider monitoring time per month (as shown in the table below). As part of the CY 2026 MPFS proposed rule, CMS is seeking comment on adopting these updated codes as policy.
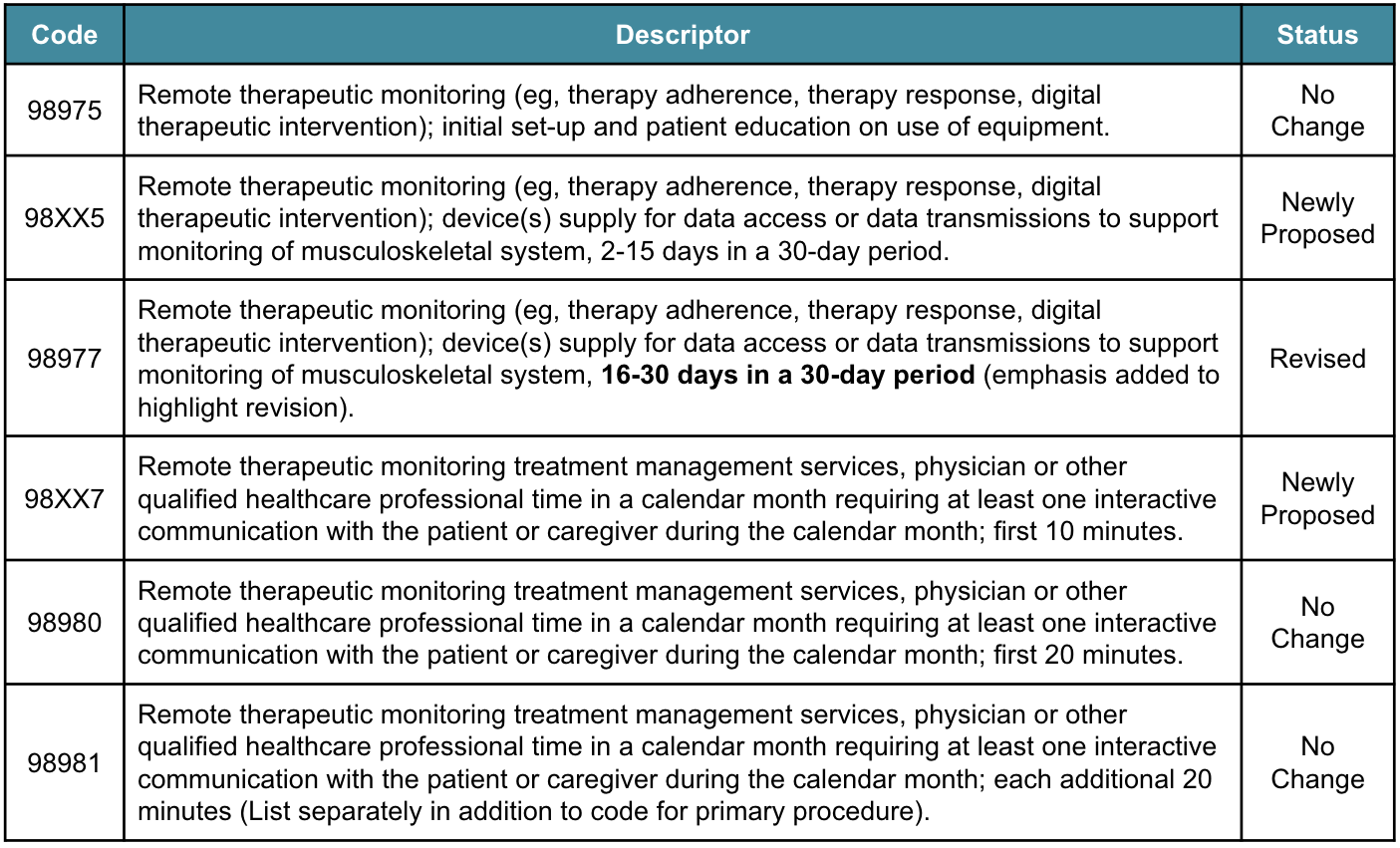
Moreover, CMS noted in this proposed rule that all RTM codes are considered new technology and will be placed on the New Technology List. This means that these codes will remain in effect for at least 3 years (until April 2030), at which time the available data will be reviewed to determine if any changes or updates are required.
Finally, while CMS rejected proposals from the Relative Value Scale Update Committee (RUC)’s recommendation to increase the Relative Value Units (RVUs) for some RTM codes, it did introduce proposals regarding payment valuation, including utilizing cost data from the Hospital Outpatient Prospective Payment System (OPPS) to value codes 98XX5 and 98977. In addition, CMS is also seeking comments on if there are differences in valuing codes for RTM in comparison to remote physiological monitoring (RPM), including variation in cost or practice expenses.
Payment Policy for Software as a Service (SaaS)
Beyond RTM, CMS is also seeking comments on how to pay for SaaS technologies going forward. Current practice expense (PE) methodologies were built on data that predates today’s digital care tools and do not fully capture costs tied to subscriptions, licensing, and algorithms.
In the CY 2026 proposal, CMS acknowledges that the lack of a consistent payment framework for SaaS is limiting provider adoption of FDA-cleared or approved technologies. To address this, CMS is soliciting public comment on:
- Whether SaaS should be valued as direct or indirect practice expense, or tied to OPPS cost data (similar to RTM and RPM).
- How SaaS fits into risk-based payment models and chronic disease management, where digital care platforms increasingly drive outcomes.
- How to value the work physicians perform in using and interpreting SaaS outputs.
- Which data sources could more accurately reflect real-world SaaS costs, beyond claims data and manufacturer invoices.
Comments Open Until September 12, 2025
The CMS CY 2026 MPFS proposed rule reflects a clear effort toward making RTM more usable for more providers; by lowering thresholds for patient engagement and provider time spend, CMS is signalling that it agrees with provider concerns that existing codes were too restrictive for widespread adoption.
Similarly, the CMS request for comment on SaaS coverage demonstrates a growing recognition from regulatory bodies that technology-enabled care is increasingly central to care delivery. A sustainable policy for SaaS reimbursement can empower broader uptake of technology that supports patients, lowers costs, and facilitates ongoing quality improvement.
Stakeholders can provide CMS with comments or request clarifications by September 12, 2025.
Schedule a call with us to learn how Force Therapeutics can support your organization’s RTM and quality improvement programs:






